
Solved Which of the following molecule is polar? O SF2 O CO2
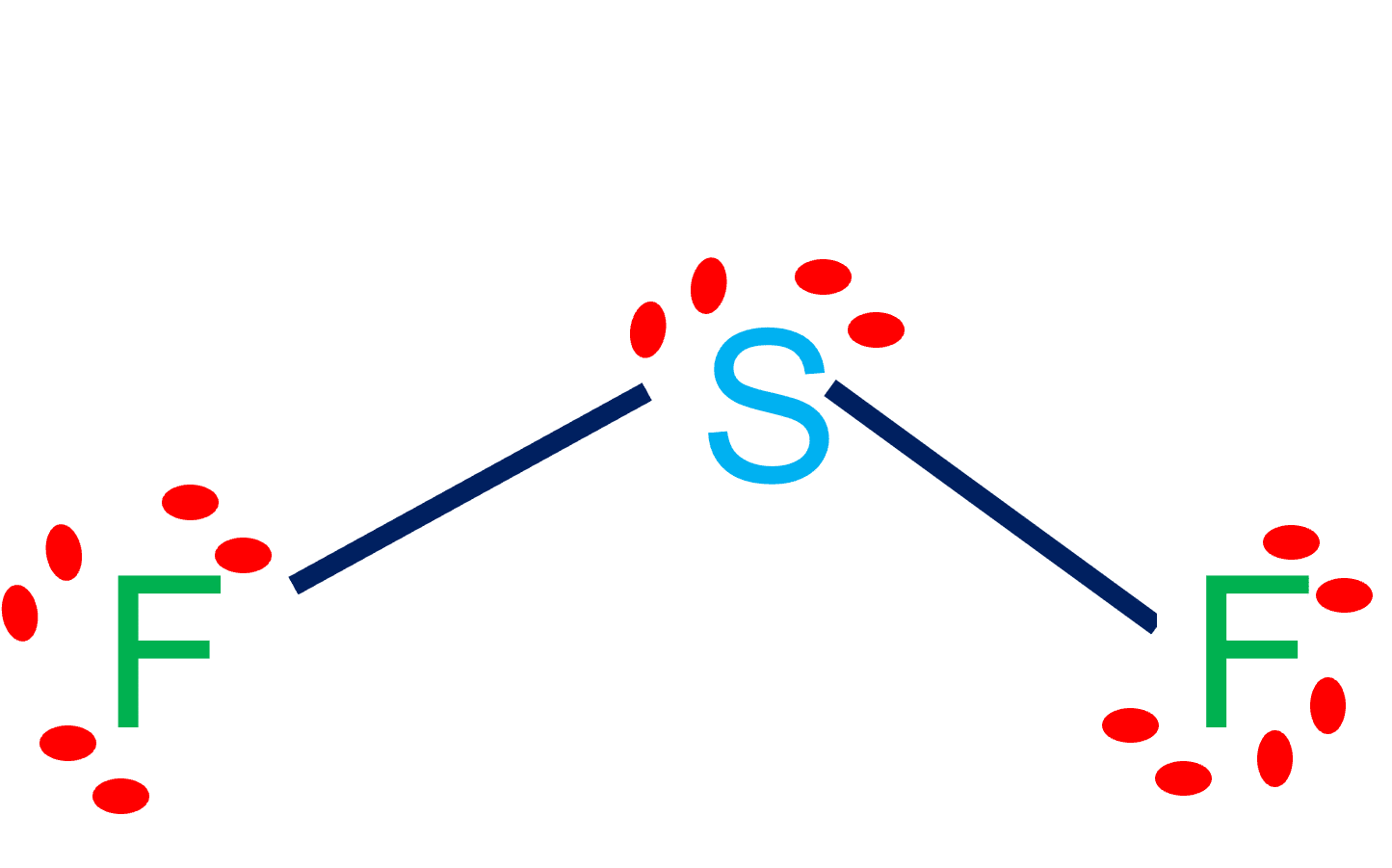
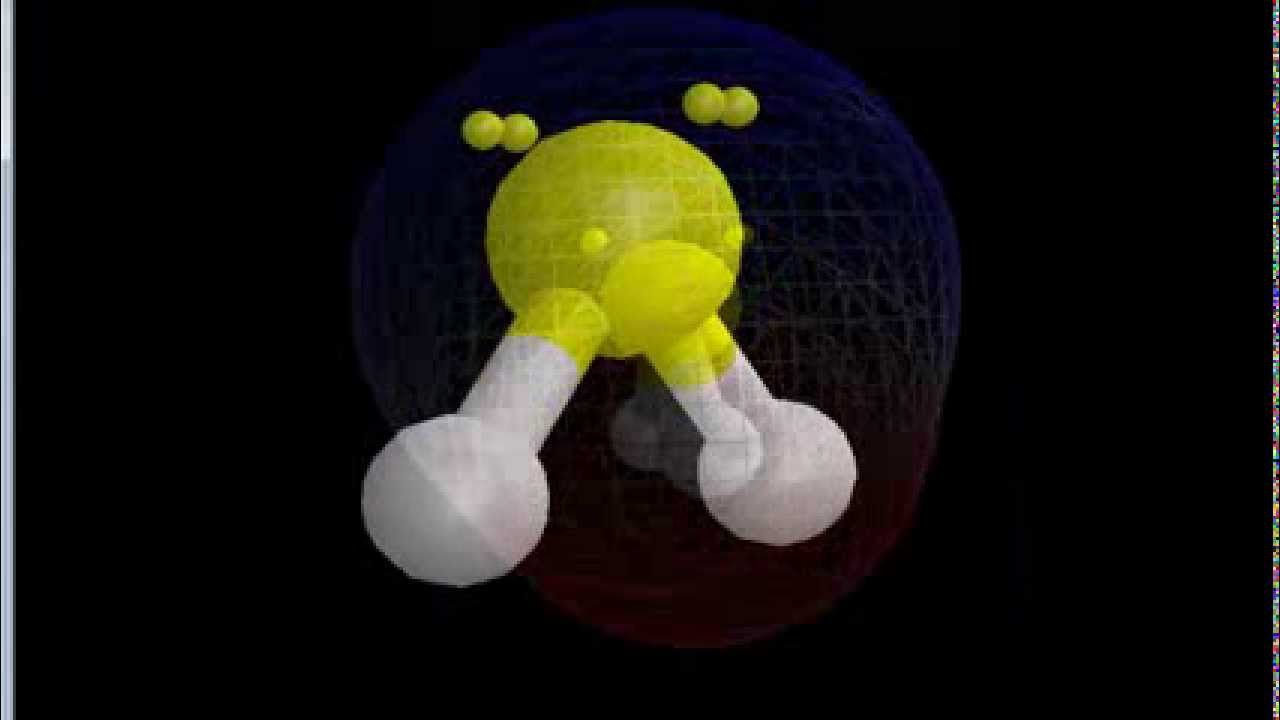
Sulfur difluoride (SF2) is a polar molecule. The central sulfur (S) atom in SF2 is surrounded by two fluorine (F) atoms forming a bent or V-shaped molecule. A fluorine (F) atom is more electronegative than a sulfur (S) atom. Thus both S-F bonds are individually polar in the SF2 molecule and possess a specific dipole moment value.
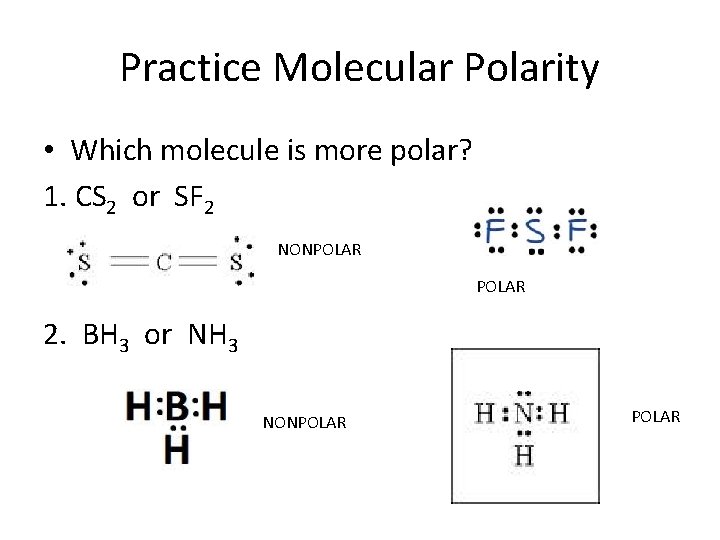
Sf2 polar or nonpolar jujapress
Is SF2 Polar or Nonpolar? (Sulfur Difluoride) - YouTube 0:00 / 1:24 Is SF2 Polar or Nonpolar? (Sulfur Difluoride) Geometry of Molecules 2.05K subscribers Subscribe 6 Share 1K views 1 year ago.

Is SF2 Polar or Nonpolar? Techiescientist
Answer. 4.10: Polar Molecules is shared under a CC BY-NC-SA 3.0 license and was authored, remixed, and/or curated by LibreTexts. The molecular polarity of a diatomic molecule is determined by the bond polarity. The polarity of molecules with more than one bond must be determined by first identifying the molecular structure and..
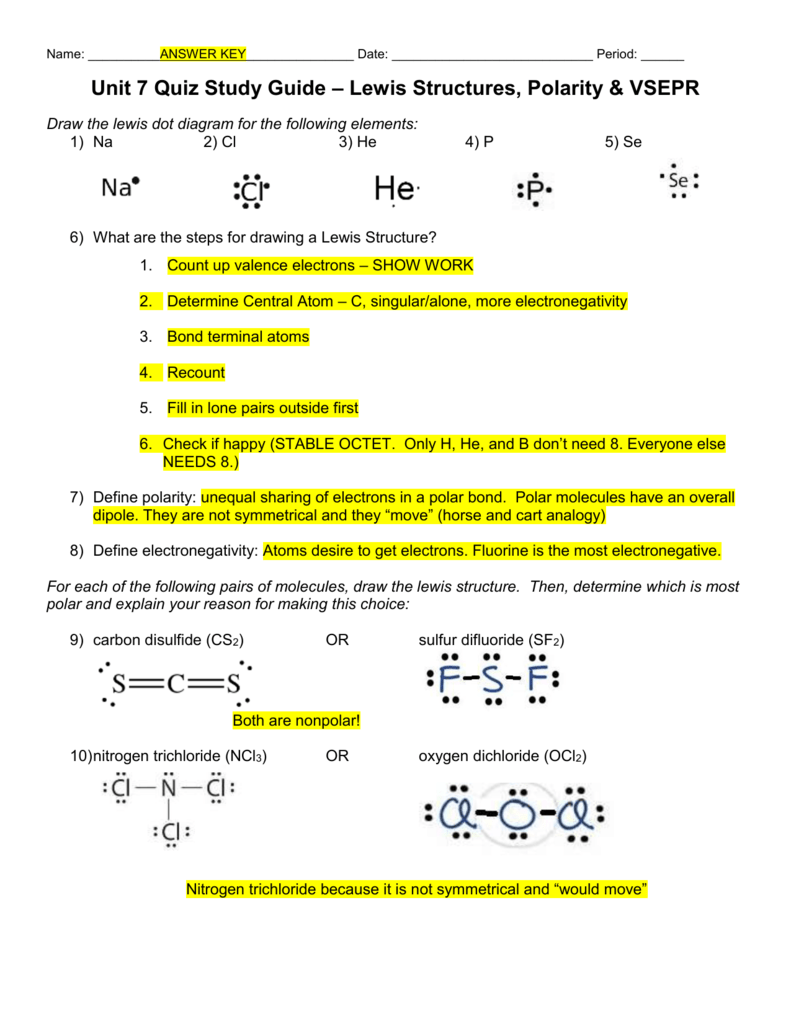
Sf2 polar or nonpolar limfath
Is SF2 Polar or Nonpolar? Wayne Breslyn 665K subscribers Subscribe 33K views 9 years ago If you look at the Lewis structure for SF2 might appear to be a symmetrical molecule. However, according.

Is SF2 Polar or Nonpolar? Techiescientist
SF2 lewis structure comprises sulphur and the most electronegative element fluorine both of which belong to adjacent groups in the periodic table. Their properties are illustrated in this article. SF2 lewis structure involves 1 sulphur and 2 fluorine atoms. The sulphur atom has 6 valence electrons and the fluorine atom has 7 valence electrons.

Is sf2 polar or nonpolar infopay
Which of these molecules and ions contain polar bonds? Which of these molecules and ions have dipole moments?SF2OpenStax™ is a registered trademark, which wa.

SF2 Lewis structure, Molecular geometry, Hybridization, Polar or nonpolar
Is SF2 polar or nonpolar? SF2 Valence electrons For drawing the Lewis structure for any molecule, we first need to know the total number of valence electrons. So we will first find out the total valence electrons for Sulphur Difluoride. Total number of valence electrons for SF2 - Valence electrons of Sulphur + Valence electrons of Fluorine

Sf2 polar or nonpolar limfamike
Sulfur difluoride (SF2) is a highly unstable inorganic compound. SF2 lewis structure comprises one Sulfur Atom and two Fluoride atoms. It is a polar molecule with bond angles of 98 degrees. Table of Contents Step by Step Construction of Lewis Structure SF2 Molecular Geometry SF2 Lewis Structure- Key Points SF2 Hybridization

Is SF2 Polar or Nonpolar? (Sulfur Difluoride) Polar, Chemical formula
Is S F 2 polar or nonpolar? Explain. Polar Molecule: There are many covalent chemical compounds that are known to be polar. This means that their molecular unit contains a net dipole moment,.

Sf2 molecule polar or nonpolar? [Expert Review]
1.44. Carbon Dioxide. 0. H. 2.20. Figure 8.8.1 8.8. 1: Both carbon dioxide and water have polar bonds, but only water is a polar molecule. Carbon dioxide is symmetric and the pull of the two oxygens on the carbon's electrons cancel out, so it is a nonpolar molecule with polar bonds.
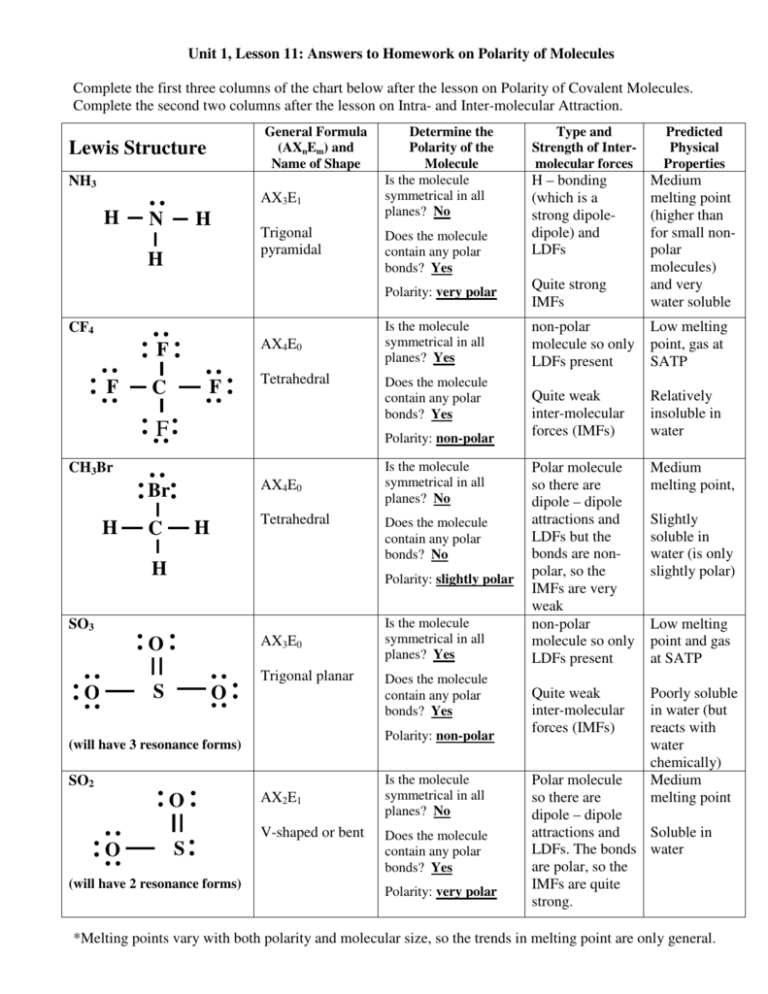
Sf2 polar or nonpolar lenaka
1. Another non polar molecule shown below is boron trifluoride, BF 3. BF 3 is a trigonal planar molecule and all three peripheral atoms are the same. Figure 4.12.1 4.12. 1 Some examples of nonpolar molecules based on molecular geometry (BF 3 and CCl 4 ). Polar molecules are asymmetric, either containing lone pairs of electrons on a central atom.
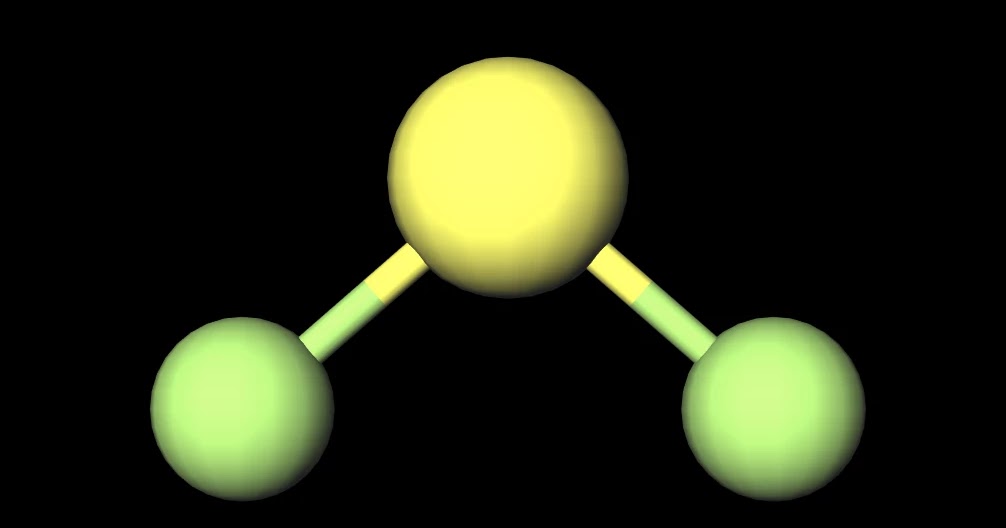
MakeTheBrainHappy Is SF2 Polar or Nonpolar?
It is an inorganic compound with the chemical formula SF2. In this article, we will discuss Sulfur difluoride (SF2) lewis structure, molecular geometry, bond angle, polar or nonpolar, its hybridization, etc. Sulfur difluoride is formed by the reaction between one mole of sulfur dichloride with 2 moles of potassium fluoride.
Solved Which of the following molecule is polar? O SF2 O CO2
SF2) molecule is classified as a polar molecule. The molecule of sulfur difluoride (with tetrahedral or bent V-shaped molecular geometry) is tilted, the bond angles between sulfur and . fluorine are 98.3 degrees. It has a difference in electronegativity values between sulfur and .

So far, we’ve used 20 of the SF2 Lewis structure’s total 20 outermost
SF2 lewis structure comprises sulphur and the most electronegative element fluorine both of which belong to adjacent groups in the periodic table. Their properties are illustrated in this article. SF2 lewis structure involves 1 sulphur and 2 fluorine atoms. The sulphur atom has 6 valence electrons and the fluorine atom has 7 valence electrons.
SF2 Lewis Structure & Molecular Geometry Simple Steps What's Insight
When you place a molecule with an electric dipole in an electric field, a force acts to turn the molecule so that the positive and negative ends line up with the field. The magnitude of the turning force is given by the formula. µ = q × d. where q is the amount of charge and d is the distance between the two charges. µ is the turning moment.
Is SF2 Polar or Nonpolar? YouTube
Sulfur Fluoride is a highly unstable inorganic compound. With a molar mass of 70.062 g/mol, this compound is made up of one Sulfur atom and two Fluoride atoms. This compound is formed when sulfur dichloride reacts at low pressure with either potassium fluoride or mercury (||) fluoride. SCl2 + 2KF —-> SF2 + 2 KCl SCl2 + HgF2 ——-> SF2 + HgCl2